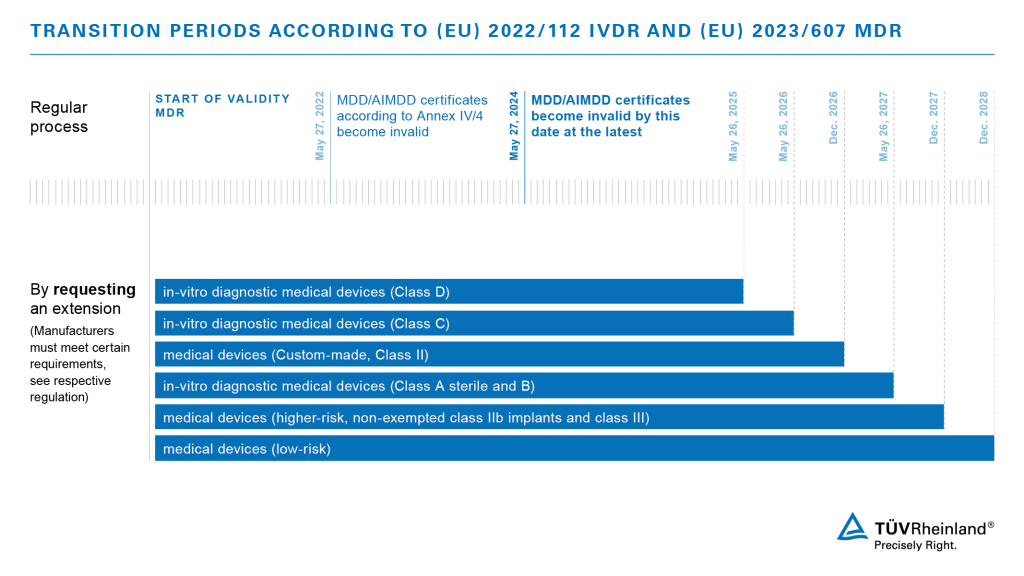
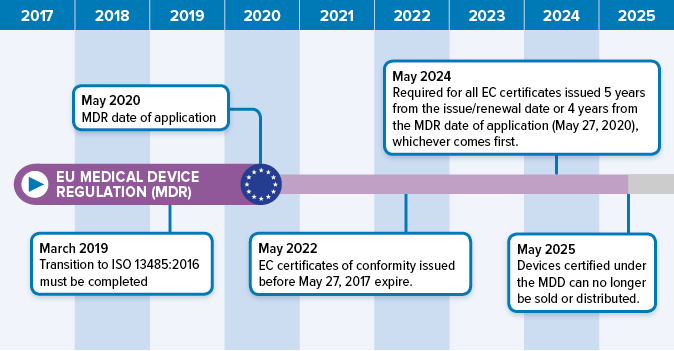
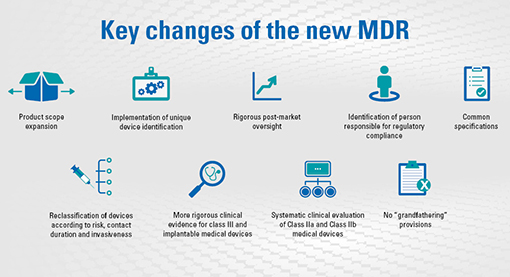
QualityCert Web App - MDR Classificator - Medical devices classification according to the EU Medical Device Regulation (EU MDR 2017/745) -QualityCert
![2021 MFDS-ACRS Conference] Drug Device Combination Products in the EU &The Impact of the New EU MDR - YouTube 2021 MFDS-ACRS Conference] Drug Device Combination Products in the EU &The Impact of the New EU MDR - YouTube](https://i.ytimg.com/vi/1-rRZ_0MkiA/maxresdefault.jpg)
2021 MFDS-ACRS Conference] Drug Device Combination Products in the EU &The Impact of the New EU MDR - YouTube
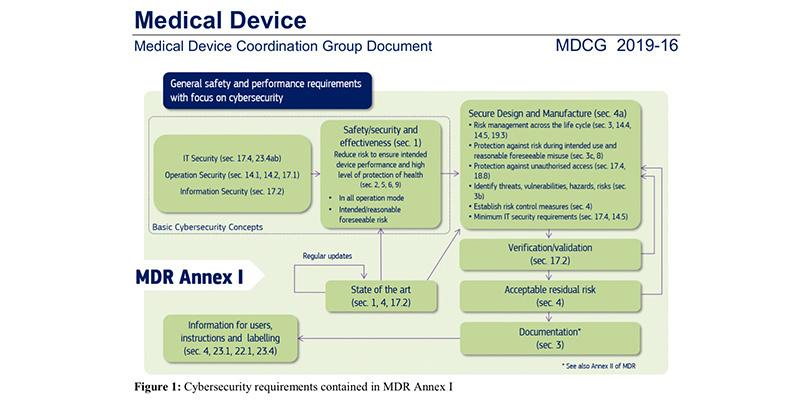
Medical Device Regulation: EU to give €100bn MedTech industry a security health check | The Daily Swig

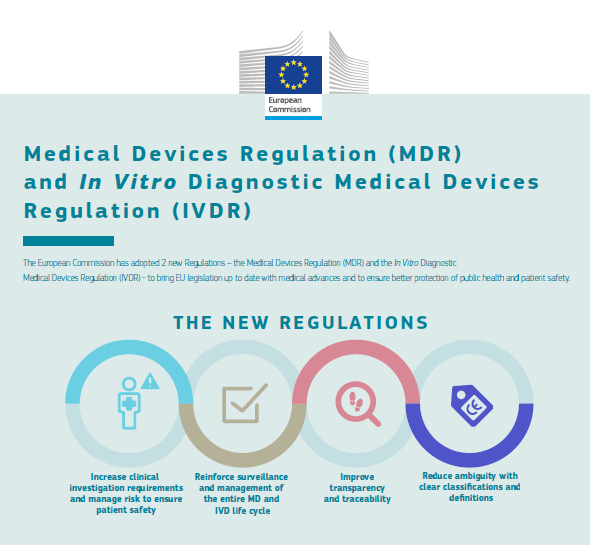
Preguntas más frecuentes sobre la nueva Regulación de Dispositivos Médicos de la UE - B Medical Systems (ES)

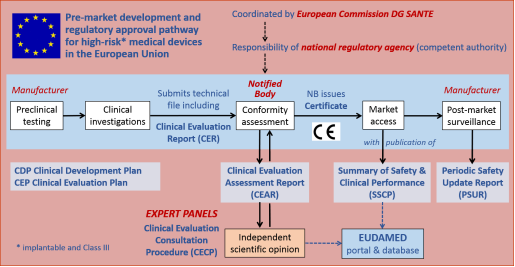
Medical device regulation in Europe – what is changing and how can I become more involved? - EuroIntervention
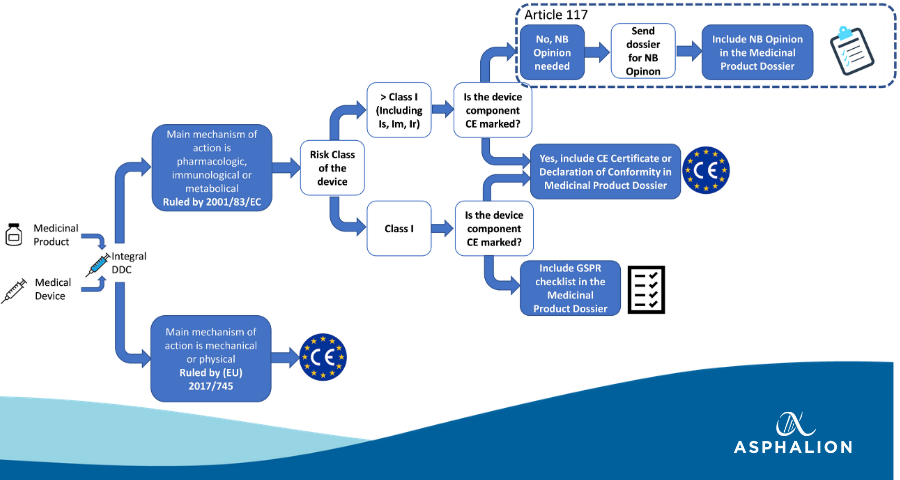
Extended Regulation of Combination Products According to Article 117 EU MDR| gempex – THE GMP-EXPERT